
Transcription factor NF-κB is over one billion years old. During this time it evolved to regulate hundreds of genes and diverse processes and cell fates ranging from proliferation, SASP, differentiation, and apoptosis. Which of these processes and which transcriptomes NF-κB will regulate depends on the cell, stimulus, timing, and context.
We study NF-κB activation across diverse cell types in health and disease, with a focus on epithelial cells of the gastrointestinal tract. Many NF-κB target genes code for secreted products such as cytokines, chemokines, and growth factors. In particular, we are interested how NF-κB regulates cellular “lexicons” – the repertoire of cell`s secreted products. This allows us to understand how epithelial cells in the gut or hepatocytes in the liver communicate with their environment.
We are using patient samples, transgenic mouse models and organoids, and rely on an array of cutting-edge interdisciplinary techniques to explore the many faces of NF-κB activation in health and disease.
Our aim is to shed light on the contribution of NF-κB and its divergent transcriptomes to development and progression of immune mediated diseases of the gut and the liver.
Ongoing projects in the lab:

1. Do bad neightbors make bad neightborhoods? Senescent cells in IBD to CRC progression: Together with our collaborators in London we are identifying and characterizing populations of senescent cells in inflammatory bowel diseases and colorectal cancer to determine how their senescent associated secretory phenotype (SASP) contributes to disease development. We aim to uncover novel therapeutic strategies by reprogramming SASP. This project is supported by the DFG grant KO 6390 1/1.
Image: Visualization of senescent cells in the gut using multiplex staining.

2. Epithelium what are you saying? Deciphering the epithelial lexicon of NF-κB in IBD: Epithelium is often overlooked as an immune organ. We are analysing the epithelial secretome and how it shapes immune cells in the gut. Our exciting recent findings point to surprising function of epithelium in guarding the gut against runaway chronic inflammation. This work is done in collaboration with our colleagues at Oxford University. This project is supported by the Fritz Thyssen Foundation and Re-Thinking Health.
Image: 3D of Intestinal organoid: stem cells shown in red, transit amplifying cells in green.

3. NF-κB in at crossroads of differentiation of secretory precursors in intestinal epithelium: We and our collaborators have recently shown that NF-κB drives differentiation of secretory precursors into Paneth cells. The mechanisms however remain unexplored. We are currently investigating how pro-inflammatory environment in colitis or IBD dictates differentiation of secretory precursors via NF-κB using scRNA, human and mouse intestinal organoids, and ChIP based techniques.
Image: NF-κB in inflammation model.
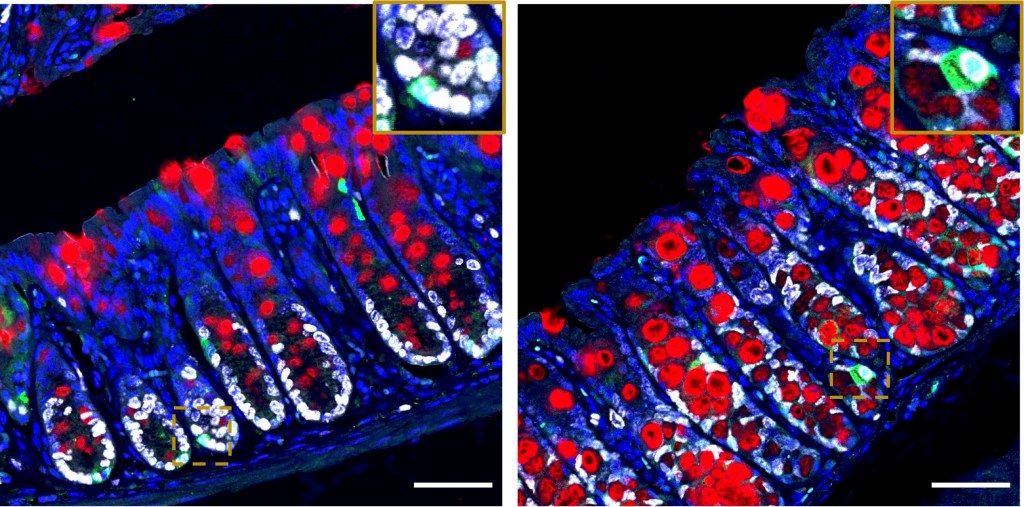
4. Deep learning to identify best fitting analgesia for colitis models: Minimising animal suffering is important for animal welfare and for reproducibility of studies. However most commonly used analgesia interferes with the exact processes studied, such as the inflammatory response. We are using deep learning together with experimental validation to identify best-fitting analgesia that alleviates pain without unwanted off-target effects. This pipeline can be effectively expanded for identification of best-suited treatments for a wide ray of pathologies. Funded by the C3R grant.
Image: Immunofluorescence of mouse gut. Normal (left) versus on analgesia.

5. Reprogramming of hepatocytes via NF-κB in ACLF: Acute on Chronic Liver Failure (ACLF) is characterized by acute decompensation of chronic liver diseases associated with organ failure. In ACLF hepatocytes lose their ability to proliferate. We are exploring the mechanisms behind the proliferative block and identifying upstream mediators to reprogram hepatocytes and bring back their proliferative potential.
Image: Hepatocytes

